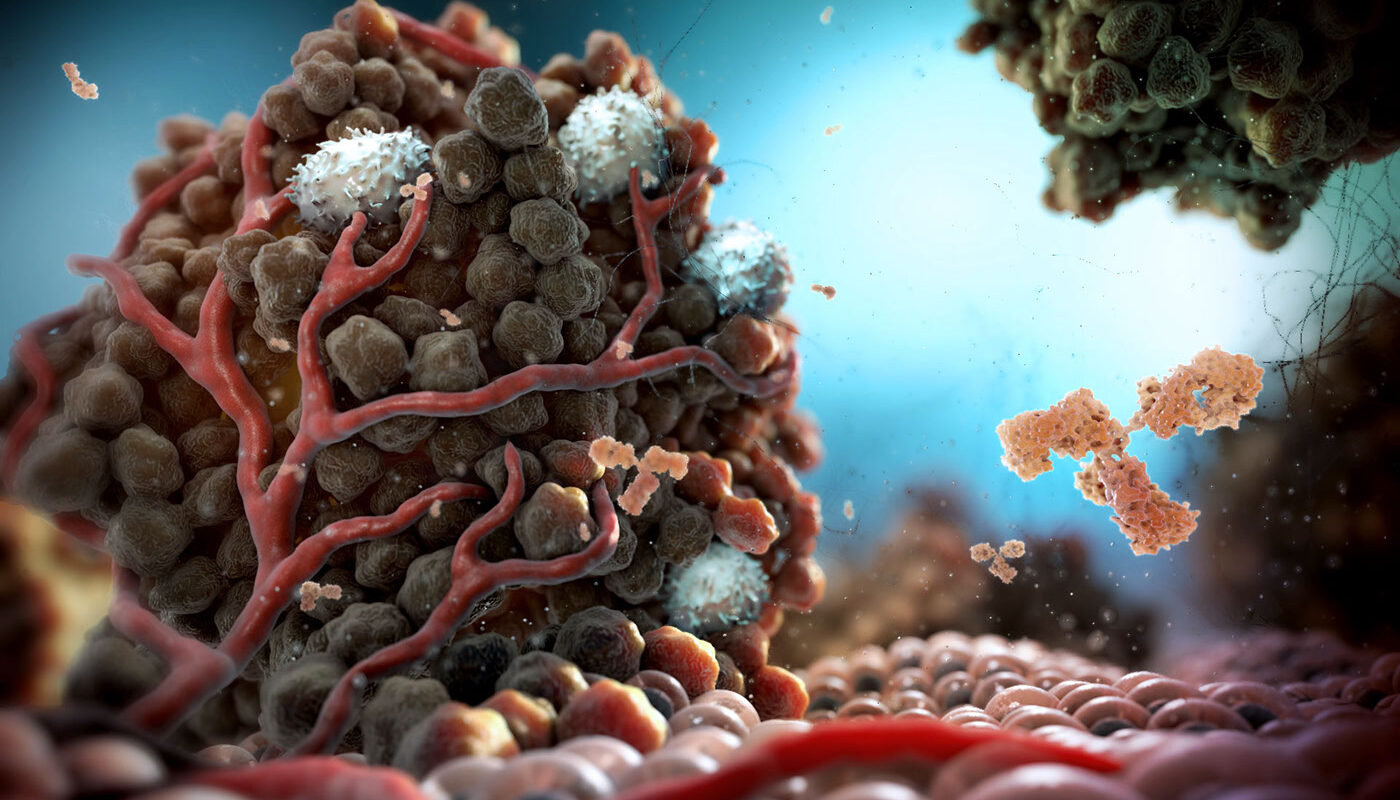
Composition of Tumor Microenvironment
The tumor microenvironment comprises the cells and molecules that surround and interact with cancer cells. In addition to cancer cells, the tumor microenvironment contains a variety of cell types such as fibroblasts, adipocytes, pericytes, endothelial cells, and immune cells. Fibroblasts are the most abundant cell type and play important roles in secreting growth factors, cytokines, and extracellular matrix proteins. Immune cells including T cells, B cells, macrophages, and mast cells migrate into tumors and influence cancer progression. Endothelial cells line newly formed blood vessels in tumors to supply nutrients and oxygen. Pericytes help maintain the integrity of these blood vessels. Adipocytes exert effects through secretion of adipokines and fatty acids. Overall, the diversity of cell types creates a complex network of interactions within the tumor microenvironment.
Impact of Tumor Microenvironment on Cancer Progression
Studies show that the Tumor Microenvironment actively participates in and promotes cancer initiation, progression, and therapeutic resistance. First, secreted factors from stromal cells influence cancer cell proliferation, survival, invasion, and metastasis. For example, fibroblasts secrete transforming growth factor beta (TGF-β), epidermal growth factor (EGF), and fibroblast growth factors (FGFs) that stimulate cancer cell growth and migration. Second, tumor-associated macrophages aid angiogenesis by secreting pro-angiogenic factors like vascular endothelial growth factor (VEGF). This supports the growth of new blood vessels to supply nutrients that fuel cancer proliferation. Third, cancer cells engage in bidirectional interactions with immune cells in the microenvironment. They may release factors that attract regulatory T cells and induce immunosuppression or alter macrophage polarization toward a pro-tumor phenotype. Together, these interactions generate a supportive niche that enables cancer cells to evade immune attack and thrive.
Altered Metabolism in Tumor Microenvironment
Metabolic alterations occur not just in cancer cells but also stromal cells within tumors. Fibroblasts undergo metabolic reprogramming to upregulate nutrient exchange pathways that cater to cancer cell needs. For example, cancer-associated fibroblasts (CAFs) display increased aerobic glycolysis and lactate release, providing an alternative nutrient source for neighboring cancer cells to utilize in oxidative phosphorylation. Moreover, adipocytes, endothelial cells, and immune cells similarly undergo metabolic changes to supply glycolytic intermediates, amino acids, and fatty acids to fuel cancer cell growth and division. This results in a metabolic cooperativity in the tumor microenvironment where non-cancerous cells function as metabolic supports for cancer cells. At the same time, tumor hypoxia and acidosis generation further shape the metabolism of stromal cells. Together, metabolic rewiring of both cancer and stromal cells helps establish a nutrient-rich microenvironment conducive for sustained cancer progression.
Role of Extracellular Matrix Remodeling
The extracellular matrix (ECM) within Tumor Microenvironment undergoes extensive remodeling induced by cancer and stromal cells. This contributes to the pro-tumorigenic nature of tumor microenvironment in several ways. First, ECM proteins like collagen, fibronectin, and hyaluronan are deposited in abnormal quantities and configurations within tumors by CAFs and cancer cells themselves. The dense, stiff matrix creates physical constraints that alter cell morphology and behavior and promotes cancer cell invasion. Second, ECM components engage specific cell-surface receptors to transduce pro-tumorigenic signals. For example, binding of collagen to discoidin domain receptors on CAFs activates them to secrete more ECM proteins and growth factors. Lastly, matrix metalloproteinases (MMPs) released by cancer and stromal cells proteolytically degrade the normal ECM structure. This promotes tumor cell migration, angiogenesis, and release of matrix-bound growth factors—all of which facilitate cancer progression and metastasis. Thus, ECM remodeling is a key pathophysiological facet of the pro-tumorigenic tumor microenvironment.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it